Purpose and objectives.
Master the method of quantitative determination of water content in petroleum products by performing fuel sample analysis.
Get acquainted with the equipment, master the technology of laboratory work. To study the design and mode of operation of devices. Learn to work with the device and determine the amount of water in petroleum products.
Laboratory equipment and materials
– Device for GOST 2477-65.
– Electric stove.
Water in petroleum products, as well as in oil, is a rather undesirable impurity, which significantly impairs their performance, causes corrosion of equipment and acts as an energy ballast when burning petroleum products. Water can get into petroleum products during storage and transportation to consumers. Permissible value of water content in petroleum products, according to their technical conditions – “traces” or complete absence.
Quantitative determination of the mass fraction of water in oil or petroleum products is carried out by the method of Dean and Stark in the device.
 In a well-dried flask (glass or metal) place a sample selected depending on the estimated water content: up to 10% – 100 ± 1 g; 10–20% – 50 ± 0.5 g; more than 20% – 25 ± 0.25. The sample of oil or petroleum product is well mixed for five minutes by shaking in a glass filled to no more than 3/4 of the container. Viscous and paraffinic oils are preheated to 40-50 ° C.
In a well-dried flask (glass or metal) place a sample selected depending on the estimated water content: up to 10% – 100 ± 1 g; 10–20% – 50 ± 0.5 g; more than 20% – 25 ± 0.25. The sample of oil or petroleum product is well mixed for five minutes by shaking in a glass filled to no more than 3/4 of the container. Viscous and paraffinic oils are preheated to 40-50 ° C.
Then measure 100 cm3 of solvent (where the flavored fraction is 80-120 ° C) into the flask, mix the contents of the flask thoroughly until the test oil is completely dissolved and add a few pieces of unglazed faience or porcelain or a few drops of silicone liquid to the flask.
Low-viscosity oils (petroleum products) are allowed to be taken in the flask by volume. In this case, the cylinder measures 100 cm3 of the test oil, pours it into the flask and, without rinsing the cylinder, measures 100 cm3 of solvent, which is also poured into the flask. The weight of oil in grams will be equal to the product of its volume by density, g / cm3.
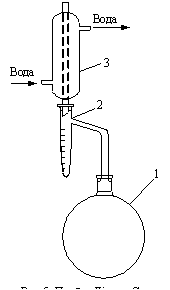
Narrow-necked flask 1 is connected directly by means of a section, and wide-necked – by means of transition and sections with a discharge tube of the pure and dry receiver-catcher 2. To the receiver-catcher connect by means of a section the refrigerator cleared by cotton wool 3. In absence of the device connections are made by means of cork stoppers. In this case, the cut end of the outlet tube of the receiver-catcher should be lowered into the flask by 1-20 mm, and the lower edge of the obliquely cut end of the tube of the refrigerator should be against the middle of the outlet tube. To avoid the passage of steam, corks are filled with collodion.
If there is a sharp difference between the room temperature and the temperature of the water entering the refrigerator, the upper end of the refrigerator tube should be covered with cotton wool to avoid condensation of atmospheric moisture inside the refrigerator tube.
The flask of the device is installed in a flask-heater or on an electric stove, heat the contents of the flask to boiling. Then the distillation is carried out so that from the obliquely cut end of the refrigerator tube into the receiver-catcher fell 2-4 drops in 1 s. If water droplets are trapped in the refrigerator tube at the end of the distillation, they are flushed into the receiver-receiver with condensed solvent, increasing the boiling point for a short time.
Distillation is stopped as soon as the volume of water in the receiver-catcher stops increasing and the top layer of solvent becomes completely transparent. The distillation time should be not less than 30 and not more than 60 minutes.
After the flask has cooled and the solvent and water in the catcher have reached room temperature, the apparatus is disassembled and water drops are removed from the walls of the refrigerator with a glass rod or wire.
If a small amount of water (up to 0.3 cm3) has accumulated in the trap receiver and the solvent is cloudy, the trap receiver is placed in hot water for 20-30 minutes to lighten and cooled again to room temperature.
Record the volume of water collected in the trap receiver to the top of one scale of the trap receiver filled with water.
The content in the test oil (petroleum product) of water in mass percent (X) is calculated by the formula
X = Vρв 100 / m
where V is the volume of water collected in the receiver-catcher, cm3;
ρв – density of water at room temperature, g / cm3 (to simplify the calculation of the density of water at room temperature is taken as 1 g / cm3, and the numerical value of water volume in cm3 – for the numerical value of water mass in g; when hanging oil 100 ± 1 g for the water content in it is taken as the number of cm3 of water collected in the receiver-catcher);
m – sample of oil taken for testing, g;
Taking the test oil in a flask with a volume of 100 cm3, the water content in it in percentage by volume is taken as the number of cm3 of water collected in the receiver-catcher. The amount of water in the receiver-receiver 0.03 cm3 or less is considered traces. The absence of water in the test oil is determined by the condition in which no drops of water are visible in the lower part of the receiver-catcher. In doubtful cases, the absence of water is checked by heating the test oil in a test tube placed in an oil bath to a temperature of 150 ° C. It is believed that water is absent when you do not hear a crack.
Differences between two parallel determinations of water content should not exceed one upper line of the scale of the receiver-catcher occupied by water.
Video of determining the water content in oil or petroleum products
